COVID-19 vaccinations will no longer be available at Medicover Medical Centres. We would like to remind that the latest information on vaccinations under the National SARS-CoV-2 Vaccination Program is also available on the dedicated website: gov.pl/szczepimysie.
|
Vaccination against COVID-19 question and answers
- How many doses of vaccine are needed to achieve immunity?
- When will my immunity develop after the vaccination?
- Is a history of COVID-19 a contraindication to vaccination?
- As a vaccinated person, can I "infect" others, can I transmit the virus?
- Should vaccinated persons still wear face masks?
- Who cannot get vaccinated?
- Why does it make sense to get vaccinated against COVID-19?
- When can you get vaccinated?
- Can the vaccine cause the coronavirus infection?
- Can the vaccine weaken my immune system and then I will fall ill, for example, with flu?
- How long does it take for the vaccine to become active? Is it true that I can still be infected with COVID-19 for two weeks?
- Will patients have to wait in the waiting room for a certain amount of time just after the vaccination?
- Is the vaccination safe for children?
- If there are side effects such as muscle pain and fever after the vaccination, how do I know that this is not a COVID-19 infection?
- If I am currently undergoing asymptomatic COVID-19, will the vaccine make me feel worse?
- Does the flu vaccine protects against COVID-19?
- Are vaccines used to implant microchips?
- Will the vaccine give me lifelong immunity?
- Will the vaccination against COVID-19 be part of Medicover packages?
- What will be the price of the vaccination against COVID-19 at Medicover?
- How can I sign up for the COVID-19 vaccination at Medicover?
- What are the side effects of the COVID-19 vaccination?
- Can I choose the vaccine I will be vaccinated with?
- Is the vaccine safe?
- Do I have to get vaccinated?
- If the vaccination causes any serious complications, will I get any compensation?
- How did it happen that the vaccine was developed so quickly?
- Will I be examined before the vaccination?
- Do any tests need to be done before the vaccination?
- What is the vaccine administration like?
- Is a referral required for the vaccination?
- Will vaccinations against COVID-19 be available at Medicover?
COVID-19 vaccination: next booster dose
The Minister of Health recommends administering the vaccine updated under the currently prevailing sub-variant XBB.1.5. The vaccination appointments will be launched from 06 December 2023.
The vaccination is recommended for:
- persons over 60 years of age;
- persons with immunodeficiency or co-morbidities that increase the risk of severe COVID-19 (applicable to individuals older than 12 years);
- healthcare professionals who have direct contact with patients or infectious material.
Where can I be vaccinated?
According to Notification no. 34 of the Minister of Health on the implementation of COVID-19 vaccination in the 2023-2024 season, the entities authorised to perform COVID-19 vaccinations from 01 December 2023 will be exclusively:
- primary healthcare (POZ) facilities,
- pharmacies open to the general public.
As a consequence of these changes, COVID-19 vaccinations will no longer be available at Medicover Medical Centres.
The current list of vaccination centres performing the vaccination from 01 December 2023 is available here: Map of vaccination centres - Vaccination against COVID-19 - Gov.pl Portal
XBB (Kraken) variant vaccine – what should we know about about it?
The protein subunit vaccine Nuvaxovid (manufactured by Novavax) is updated in line with the characteristics of the currently predominant subvariant XBB.1.5. and will also be available to those who wish to be vaccinated and meet the age requirement (over 12 years old) and at least 6 months have elapsed since their last COVID-19 vaccination.
For persons aged 12+ who were not previously vaccinated against COVID-19, the second dose should be administered in three weeks after the first dose.
The recommended interval for the next booster dose after the last booster dose in the 12+ age group is at least 6 months.
Full text of communication issued by the Minister of Health on COVID-19 vaccinations in the 2023-2024 season:
1. How many doses of vaccine are needed to achieve immunity?
Two doses of vaccine are required for Comirnaty (Pfizer), Spikevax (Moderna) and Vaxzevria Vaccine (AstraZeneca). The second vaccination is given: 21 to 42 days after the first dose for Comirnaty; 28 to 42 days after the first dose for Spikevax; 35 to 84 days for after the first dose for Vaxzevria. The COVID-19 Vaccine Janssen (J&J) is a single-dose preparation.
Booster dose of the COVID-19 vaccine
Persons older than 5 years of age who have been fully vaccinated will be eligible to get a booster shot of Pfizer or Moderna vaccines, depending on whether Comirnaty (Pfizer) or Spikevax (Moderna) was administered as the primary vaccine.
In the age group of 5-11 years a booster dose can be administered 6 months after the last COVID-19 vaccination, while among those over 12 years of age, a booster dose can be administered 3 months after the last COVID-19 vaccination. Vaccination with a booster dose in children under 15 years of age can only be administered with the Comirnaty (Pfizer).
Vaccination in people with the impaired immune system
From the beginning of September, the third booster dose of coronavirus vaccine can already be administered to people with immunodeficiencies who have reached the age of 5 and have been fully vaccinated (two doses) with Pfizer, Moderna or AstraZeneca.
Individuals eligible to get a booster dose:
- receiving active anti-cancer treatment,
- after organ transplants taking immunosuppressive drugs or receiving biological treatment,
- after stem cell transplant within the last two years,
- with moderate or severe primary immunodeficiency syndromes,
- HIV-infected,
- currently treated with high doses of corticosteroids or other immunosuppressive drugs,
- undergoing long-term dialyses for renal failure.
In the indicated immunocompromised groups, not sooner than 28 days after the second shot of COVID-19 vaccination, an additional dose is given:
- to individuals aged ≥5 years – Comirnaty (Pfizer)
- to individuals aged ≥12 years - Comirnaty (Pfizer),
- to individuals aged ≥18 years - Comirnaty (Pfizer) or Spikevax (Moderna) vaccine.
2. When will my immunity develop after the vaccination?
For the Comirnaty preparation, the vaccine may not provide full protection until at least 7 days after receiving the second dose, and at least 14 days for COVID-19 Vaccine Moderna and COVID-19 Vaccine Janssen.
The efficacy of Comirnaty, assessed as the incidence of COVID-19 at least 7 days after receiving the second dose of vaccine, was determined to be 95%. The efficacy was independent of age, sex, race, ethnic group, obesity and other existing health conditions. Between the first and the second doses, the vaccine efficacy was determined to be 52%. For COVID-19 Vaccine Moderna, the efficacy to prevent COVID-19 within 14 days after the second dose was 94.1%. For COVID-19 Vaccine AstraZeneca, partial protection starts about 3 weeks after the first injection and lasts for up to 12 weeks. Vaccine efficacy in reducing the incidence of disease was estimated to be approximately 60% from day 15 after taking the second dose.
For the AstraZeneca COVID-19 Vaccine, the efficacy in preventing COVID-19 ≥15 days after the second dose was approximately 60%.
For the Janssen COVID-19 Vaccine, the efficacy in preventing COVID-19 was assessed 14 days after vaccination and the results are as follows:
- 9% in the analysis of all participants,
- 2% in people aged 18-64,
- 4% in people aged 65 and more,
- as much as 100% in people aged 75 and over.
In turn, 28 days after vaccination, it was 66.1% in the analysis of all participants, 65.1% in those aged 18-64, and 74% in those aged 65 and over.
Efficacy against severe COVID-19 was 76.7%, assessed 14 days after vaccination and 85.4%, 28 days after vaccination.
The currently available vaccines may not provide protection to all vaccinated individuals.
The duration of protection provided by the vaccines is unknown as this is still being determined in ongoing clinical trials.
The effectiveness of Comirnaty and Vaxzevria vaccine in preventing symptomatic COVID-19 disease caused by the Delta variant was just slightly lower than that of the Alpha variant. The greatest protection was provided by two doses of the vaccine.
The effectiveness in preventing symptomatic COVID-19 caused by the Delta variant was as follows (data from https://szczepienia.pzh.gov.pl/jaka-jest-skutecznosc-szczepionek-przeciw-covid-19-wobec-wariantu-delta-wirusa-sars-cov-2/, accessed 09/23/2021):
- 9% (95% CI: 78.2–93.2) for Comirnaty vaccine (assessed ≥14 days after the second dose) and was similar to the effectiveness in cases caused by the Alpha variant of 93.4% (95% CI: 90.4-95.5);
- 8% (95% CI: 28.9-77.3) for Vaxzevria vaccine (assessed ≥14 days after the second dose) and was similar to the effectiveness in cases caused by the Alpha variant of 66.1% (95% CI: 54-75);
- 2% (95% CI: 8.3-51.4) for Comirnaty vaccine and 32.9% (95% CI: 19,3-44.3) for Vaxzevria vaccine (assessed ≥21 days after the first dose); the effectiveness against the Alpha variant was slightly higher at 49.2% (95% CI: 42.6-55) for Comirnaty vaccine and 51.4% (95% CI: 47.3-55.2) for Vaxzevria vaccine.
3. Is a history of COVID-19 a contraindication to vaccination?
No, according to the US Center for Disease Prevention and Control (CDC), the vaccine can be safely administered to people who have a history of COVID-19.
According to the regulation of 11 March 2021, people who have had COVID-19 can be vaccinated against COVID-19 not earlier than 30 days after obtaining a positive test for the presence of the SARS-CoV-2 virus.
Since people with SARS-CoV-2 infection have already acquired immunity, and relapses of COVID-19 within 90 days of the original infection are rare, vaccination of convalescents may be postponed even until then.
This does not apply to people who:
- are on dialysis for chronic renal failure,
- are undergoing cancer treatment with chemotherapy or radiotherapy conducted after 31 December 2019,
- are under chronic mechanical ventilation,
- have had cell, tissue and organ transplants and underwent immunosuppressive therapy,
- have been diagnosed with cancer and qualified for chemotherapy or radiotherapy treatment, but have not started treatment yet,
- are waiting for a transplant.
Vaccinating patients with an ongoing SARS-CoV-2 infection should be postponed.
4. As a vaccinated person, can I "infect" others, can I transmit the virus?
Vaccination protects against severe infection or hospitalization, but it does not exclude the possibility of transmitting the virus and infecting unvaccinated individuals with it. A clinical trial looked at the effect of vaccination in preventing symptomatic COVID-19, but not the SARS-CoV-2 infection. A vaccinated person can become infected after contact with a sick person, and although they will not develop the disease, they can infect others. It is not yet known whether vaccines also protect against the spread of the virus.
5. Should vaccinated persons still wear face masks?
Experts recommend that the vaccinated individuals should continue to cover their mouths and noses, maintain the recommended social distance, avoid gatherings, and wash or disinfect their hands frequently. The vaccination protects against the disease, but does not rule out transmission of the virus and infecting unvaccinated people. It is not yet known how vaccines are going to stop the spread of SARS-CoV-2 infection, so every available means of limiting the range of this pandemic should be used. Information on policies for vaccinated persons should be available at government websites.
6. Who cannot get vaccinated?
Hypersensitivity to the active substance or to any of the substances included in the vaccine is an absolute contraindication to vaccination, including in individuals who have experienced an anaphylactic reaction after the first dose of COVID-19 vaccine or a severe allergic reaction to the active substance or another vaccine component. Before vaccination, please inform the doctor if you have ever had a severe allergic reaction to any medication, vaccine or food.
During the qualification for the vaccination, inform the medical staff if you:
- suffer from any allergies,
- have a fever,
- have a blood clotting disorder and take anticoagulants,
- have a reduced immunity or take any medication that affects your immune system,
- are pregnant (or plan to get pregnant),
- are breastfeeding,
- have already been vaccinated with another vaccine against COVID-19.
Temporary contraindications to vaccination are as follows:
- current or recent SARS-CoV-2 infection (until recovery) – this is to avoid the misallocation of any new symptom or progression of symptoms to the vaccine. As some people with COVID-19 may experience deterioration up to 2 weeks after infection, the vaccination should be postponed until recovery and at least 4 weeks after the onset of symptoms or 4 weeks after the first positive PCR test in case of asymptomatic carriers;
- acute, severe infection with an accompanying fever – until the symptoms of infection disappear.
There is no contraindication for vaccinating individuals with immune disorders or receiving immunosuppressive treatment – but they may have a reduced immune response. The final decision on whether such a person can be vaccinated will be made by a doctor who qualifies for the vaccination.
Special care must be taken in case of patients with clotting disorders and patients taking anticoagulants. The doctor needs to be informed about any history of clotting disorder and about taking any medications for this condition. After the vaccination, some bleeding or haematoma may occur.
Vaccination of recovered patients
Studies to date have not confirmed any concerns about the safety of vaccinating people with a history of COVID-19 infection or with detectable COVID-19 antibodies. Consequently, the recovered patients should also be vaccinated. This is because it is not yet known how long the antibodies produced in response to the natural infection persist and whether the vaccination provides better protection. It is supposed that if the antibodies have already been produced as a result of a natural infection, getting infected with COVID-19 will activate the immune system.
Post-vaccination side effects
The majority of side effects are mild and should not last longer than a week. These side effects include, for instance:
- short-term mild to moderate pain at the site of vaccination,
- tiredness,
- headache,
- muscle pains,
- shivers,
- joint pains,
- fever
- redness or swelling at the vaccination site,
- nausea,
- poor physical state,
- lymph node enlargement.
The safety of the vaccine was tested two months after the second dose was administered. However, observations of adverse reactions will continue to be made – as with any other medicinal product.
7. Why does it make sense to get vaccinated against COVID-19?
The benefits of the vaccination:
- Taking Comirnata vaccine and Spikevax protects you in 95% against COVID-19 and serious complications of infection. The effectiveness of COVID-19 Vaccine AstraZeneca in protecting against COVID-19 has been estimated at approximately 60%.
Getting vaccinated can help prevent the severe course of COVID-19 even if you get the disease.
There was no person with a severe course of COVID-19 in the group receiving COVID-19 Vaccine AstraZeneca Spikevax. There are no such data reported for Comirnata vaccine. - The vaccination helps the body to develop resistance to the virus that causes COVID-19 without first getting sick.
- You will protect others – family and friends, especially all persons with an increased risk of
a serious disease caused by COVID-19. - You will help to reduce the spread of the pandemic. Wearing masks and keeping the social distance helps to reduce the risk of exposure to the virus or spreading it to others, but these measures are not enough. If 50% of the population gets vaccinated, the spread of coronavirus will be significantly reduced. And by reducing the pandemic, it will be possible to slowly lift the restrictions and return to normal life.
- Your example can encourage others - your family or friends.
- Vaccinated people may skip quarantine.
- Vaccinated people will not be counted towards the limit of five person for home events.
- Vaccinated people can freely participate in rehabilitation.
- Vaccinated people will not have to present a negative coronavirus test result when applying for admission to treatment or nursing facilities, social welfare home, inpatient hospice or palliative medicine unit.
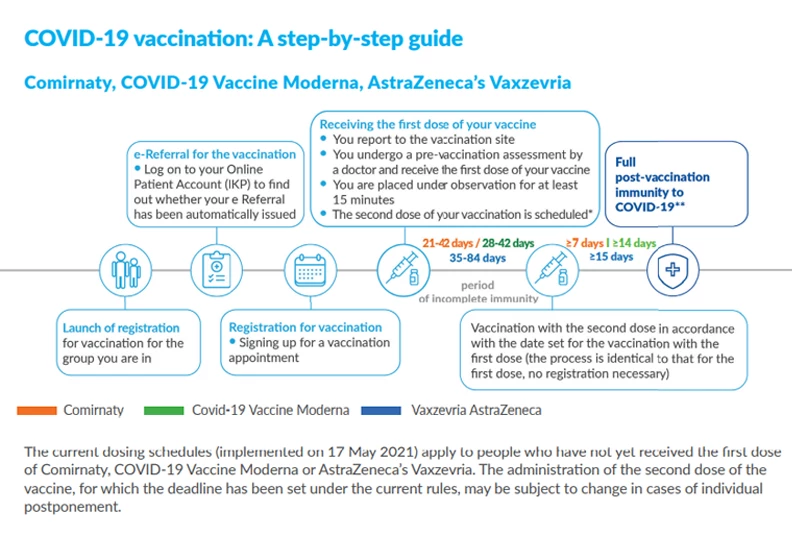
8. When can you get vaccinated?
Vaccinating adolescents over 16 years of age
Starting 17 May, registration was opened to those who turned 16 by 17 May and for all people born in 2004.
For people born in 2005, e-Referrals will be issued at the age of 16 – every Monday. At that point it will be possible to sign up for COVID-19 vaccination.
People born in 2003 received an automatic referral on 17 May and can register for the COVID-19 vaccination.
The pre-vaccination questionnaire for 16- and 17-year-olds is completed by both the vaccinated person and the adolescent's parent or legal guardian. If the questionnaire and consent are completed and signed at home, then the parent's presence at the time of vaccination is not necessary.
Vaccination for 12-15-year-olds
From June 7th, parents can register their children aged 12-15 for vaccinations against COVID-19. The e-Referral can be found on the Internet Patient Account of the child. Children aged 12-15 are vaccinated with Comirnaty or Spikevax by Moderna.
The pre-vaccination questionnaire for 12-15-year-olds is completed by both the vaccinated person and their parent or legal guardian. If the questionnaire and consent are completed and signed at home, the parent's presence at the vaccination is not necessary.
Vaccination for 5-11-year-olds
From December 14, 2021, parents can register their children aged 5-11 for vaccinations against COVID-19. Children aged 5-11 are vaccinated with Comirnaty from Pfizer / BioNTech.
Prior to vaccination, pre-vaccination questionnaire should be completed to provide an applicant with a basis for whether or not a child can be immunized at that date.
The doctor decides about the eligibility of children aged 5-15 for vaccination each time.
All adults and children over 5 years of age are eligible to receive a booster dose of COVID-19 vaccine.
Vaccinations for foreigners
How to get vaccinated against COVD-19 if you don't have a PESEL number?
Vaccinations against COVID-19 for foreigners legally residing in Poland are provided free of charge.
Regardless of whether you are:
- a person learning or studying in Poland,
- a foreign doctoral student,
- a foreigner with a work permit,
- a diplomat,
- a spouse or child of one of the abovementioned persons with the right to reside in Poland
you should see a primary care physician who will issue a referral based on your identity document.
With the e-Referral issued, you can sign up for vaccination in one of two ways:
- by calling the Medicover helpline at 500 900 530,
- by calling the helpline at 989,
- directly at the selected vaccination point.
When signing up for vaccination, enter the number of the document that appears on the e-Referral.
Please bring with you to the vaccination the identity document that your doctor used to enter the information. Remember to use the exact same identity document that was used to issue the e-Referral at each stage of the vaccination process.
9. Can the vaccine cause the coronavirus infection?
No, because available vaccines contains only genetic information (mRNA) about the structure of a single SARS-CoV-2 virus protein. After the vaccination, the body "reads" this information and, on its basis, starts to produce antibodies. The information itself (mRNA) is not capable of causing an infection.
10. Can the vaccine weaken my immune system and then I will fall ill, for example, with flu?
No. The basis of the work of the immune system is made of millions of lymphocytes prepared to fight millions of antigens at the same time, which at any time come into contact with our skin, nasal or mouth mucous membranes and the interior of the digestive tract. The vaccine carries a very small dose of the antigen, so only a small proportion of the immune system cells are involved. Other cells continue to fight against the microorganisms they encounter, such as influenza viruses.
11. How long does it take for the vaccine to become active? Is it true that I can still be infected with COVID-19 for two weeks?
Comirnata vaccine is fully effective starting from 7 days after the second dose, only partial protection is gained after the first dose. In the case of Spikevax – this time period is at least 14 days.
COVID-19 Vaccine AstraZeneca may not provide complete protection until at least 15 days after receiving the second dose of vaccine. In turn, the Janssen COVID-19 vaccine may not provide full protection up to 14 days after administration (one dose only). Currently available vaccines may not protect everyone who is vaccinated.
If the contact with an infected person takes place between the first and second dose, it is possible to get sick. If symptoms of infection appear, contact your doctor.
12. Will patients have to wait in the waiting room for a certain amount of time just after the vaccination?
Yes, it is recommended to stay at the healthcare centre for 15 minutes after the vaccination has been administered.
13. Is the vaccination safe for children?
In Poland, parents can sign up their children from the age of 5 for vaccination. Children are vaccinated with Comirnaty (Pfizer/BioNTech), which are the only vaccines approved by the European Medicines Agency for use in children of this age.
Prior to the vaccination, a pre-vaccination screening questionnaire should be filled out to inform the qualifying HCP if your child can be vaccinated on a given date. The pre-vaccination questionnaire for 16-year-olds and 17-year-olds should be filled out by both the vaccinated person and the teenager’s parent or legal guardian. When it comes to children aged 5-15, the questionnaire is filled out only by the parent or legal guardian. If the questionnaire and consent are filled out and signed at home, the parent does not have to be present in the treatment room during their child’s vaccination. For up-to-date information, please visit the government website.
14. If there are side effects such as muscle pain and fever after the vaccination, how do I know that this is not a COVID-19 infection?
If after the vaccination you have symptoms such as a fever or cough, you should consult your doctor. If the doctor thinks it is likely that you have COVID-19, you will be tested.
15. If I am currently undergoing asymptomatic COVID-19, will the vaccine make me feel worse?
No – and moreover, scientists assume that if the antibodies have already been produced as a result of an infection, receiving the vaccine stimulates the immune system. However, there is currently no data to support this theory.
16. Does the flu vaccine protects against COVID-19?
No – the flu vaccine only protects against the flu virus.
17. Are vaccines used to implant microchips?
The vaccines do not contain any microchips or other harmful elements. The vaccines are medicinal products (just like medicines), so their composition is rigorously controlled in the production of each batch of preparations. This ensures that their composition is identical to what the leaflet says.
18. Will the vaccine give me lifelong immunity?
Research and observations of the duration of post-vaccine immunity are still ongoing. The data as of 23 September 2021 show that the amount of antibodies protecting against the disease decreases over time. Now (from September 2021), the government has modified the vaccination programme to include booster shots of vaccine for specific groups of people (see section 1).
19. Will the vaccination against COVID-19 be part of Medicover packages?
Medicover patients, just like all adults in Poland, can be vaccinated free of charge as part of vaccination programme financed by the state budget. Anyone who has no contraindications to vaccination will be able to get vaccinated for free. Consequently, vaccinations against COVID-19 will not be part of Medicover packages.
20. What will be the price of the vaccination against COVID-19 at Medicover?
The vaccination against COVID-19 is free of charge and financed by the state budget for any citizen who wants to get vaccinated and does not have any contraindications to vaccination.
21. How can I sign up for the COVID-19 vaccination at Medicover?
You can sign up for vaccination in one of three ways:
- by calling the Medicover helpline at 500 900 530,
- by calling the helpline at 989,
- directly at the selected vaccination point.
22. What are the side effects of the COVID-19 vaccination?
Clinical trials revealed the occurrence of adverse effects typical of vaccines. The majority of such adverse reactions (side effects) are mild and should not last longer than a few days. Serious adverse post-vaccination reactions occurred rarely. The frequency of severe allergic reaction (anaphylaxis) and hypersensitivity is not known.
The occurrence of systemic adverse post-vaccination reactions was more common after the second dose than after the first of the vaccine, and their course was also more severe. The frequency of adverse post-vaccination reactions of local nature was similar after the administration of both doses.
| The frequency of adverse post-vaccination reactions | |||||
Very common (may occur in more than 1 out of 10 individuals) | Common (may occurin maximum 1 out of 10 individuals) | Uncommon (may occur | Rare (may occur | Frequency unknown(cannot be estimated from the data available) | |
| Comirnaty (Pfizer/ BioNTech) |
|
|
|
| - |
| COVID-19 Vaccine Moderna |
|
|
|
| - |
| COVID-19 Vaccine AstraZeneca |
|
|
| - | - |
| COVID-19 Vaccine Janssen |
|
|
|
| ● anaphylaxis |
Very rare post-vaccination reactions of COVID-19 Vaccine AstraZeneca and COVID-19 Vaccine Janssen are blood clots with thrombocytopenia.
23. Can I choose the vaccine I will be vaccinated with?
Theoretically, as the vaccination against COVID-19 is free of charge and financed by the state budget, it is not possible to choose the preparation.
However, as the supply of vaccines increases, sometimes during registration you may find that you have a choice of vaccine type. It depends on your location and actual deliveries.
The exception are children aged 5-11 years who can only get vaccinated with Comirnaty by Pfizer - so far the only one approved in this age group in Poland.
24. Is the vaccine safe?
The safety of the vaccine is one of the basic criteria that the European Medicines Agency follows when issuing a decision on its authorisation for use in the European Union. Other criteria include the efficacy and quality of the vaccine as well as an assessment of the benefit-risk balance of the vaccine.
All currently authorized vaccines (Comirnaty, COVID-19 Vaccine Moderna and COVID-19 Vaccine AstraZeneca) mainly cause mild vaccine adverse reactions, i.e. pain at the injection site, fatigue, fever, headache. Most vaccine adverse reactions were mild to moderate in severity and usually resolved within a few days of vaccination.
The safety of Comirnata vaccine and COVID-19 Vaccine Moderna was investigated up to 2 months after the second dose. For COVID-19 Vaccine AstraZeneca, the median follow-up after the second dose was 62 days.
As with any other medicinal product, observations for adverse reactions for all vaccines are continued.
25. Do I have to get vaccinated?
For the sake of your health, we recommend to do so.
26. If the vaccination causes any serious complications, will I get any compensation?
The government has announced that a special fund was set up. The fund will be used to pay compensation to individuals who have suffered adverse vaccination reactions.
27. How did it happen that the vaccine was developed so quickly?
First of all, the technology that many scientists have been working on for years has been used – and the research on the use of mRNAs in vaccines has been going on for at least several years. One of the latest such vaccines on which scientists worked was the vaccine against the Zika virus. Research is also under way on such a vaccine against influenza.
Moreover, a great deal of work, research and legislation has been carried out at the same time, and agencies responsible for authorising medicines on the market (such as the European EMA and the American FDA) have allowed for the documentation to be delivered in batches. In addition, many official deadlines for submitting the relevant documents or issuing opinions have been reduced.
Although the whole process has been sped up, however, all the standard procedures relating to the quality, safety and efficacy of the vaccine have not been ignored.
28. Will I be examined before vaccination?
Yes, before the vaccination the medical staff carries out a qualification. During the qualification please inform if you:
- suffer from any allergies,
- have a fever,
- have a blood clotting disorder and take anticoagulants,
- have a reduced immunity or you are taking medications that affect your immune system,
- are pregnant (or plan to get pregnant),
- are breastfeeding,
- were vaccinated with another vaccine against COVID-19.
Before the vaccination of children under 15 years of age, the qualification is carried out only by a doctor.
29. Do any tests need to be done before the vaccination?
No, vaccination does not require any special preparation or tests. Right before the vaccination actually takes place, there is a qualification at which you have to arrive with a pre-vaccination screening questionnaire – it is the basis for the qualifying HCP to administer the vaccine.
30. What is the vaccine administration like?
First, there will be a qualification for vaccination. Then, a nurse will administer the vaccine in your arm. The second dose (21 days for Comirnaty by Pfizer, 28 days for Spikevax by Moderna or 70 to 84 days for Vaxzevria by AstraZeneca after the first dose) will be given in the same way.
There are several doses of the vaccine in one vial, so the nurse will take the right amount of liquid into a sterile disposable syringe, and then will proceed to make an injection.
31. Is a referral required for the vaccination?
The vaccination process will be based on an invitation (an e-referral document) that is valid for 60 days from its issue date. E-referrals will be generated automatically in the P1 system (Electronic Platform for Collection, Analysis and Sharing of Digital Medical Records) in batches to make sure they are consistent with the order of vaccinations (for specific age groups and specific professionals, etc.). Moreover, doctors will be able to issue individual e-referrals for patients (e.g. for an individual who does not have a PESEL number, or for a person who could not be vaccinated during the period of validity of their first e-referral).
32. Will vaccinations against COVID-19 be available at Medicover?
Yes, Medicover will participate in the process of vaccinations against COVID-19. A list of Medicover facilities where you can be vaccinated against COVID-19 is available.
The material was prepared on the basis of sources valid as at 06 October 2022. The information will be updated.